Microflow G (GMP system)
Microflow G (GMP system)
As a clinical manufacturing device which is design to conform GMP regulation, Microflow G has the same large synthesis capacity up to 120 L/h as Microflow M and moreover possesses most certificate for GMP regulation, including sterility, material, dissolution, and etc. Extra certificate requested by customers can also be performed. Large range of flow rate ratio (1:1 to 10:1) and reusable design for cartridge is reserved, but single-use for clinical manufacturing is recommended.
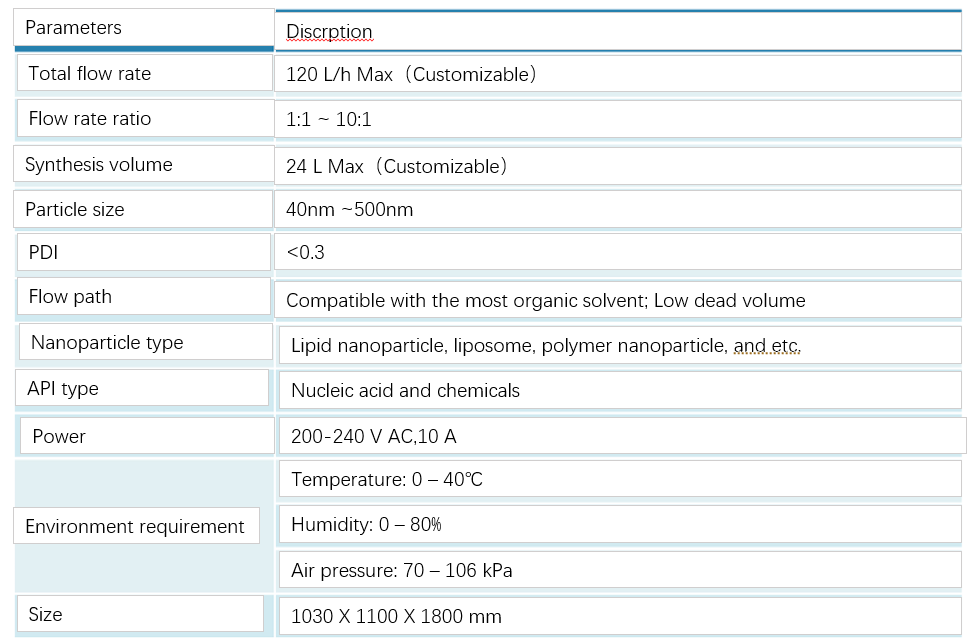
Parameters (Microflow G)
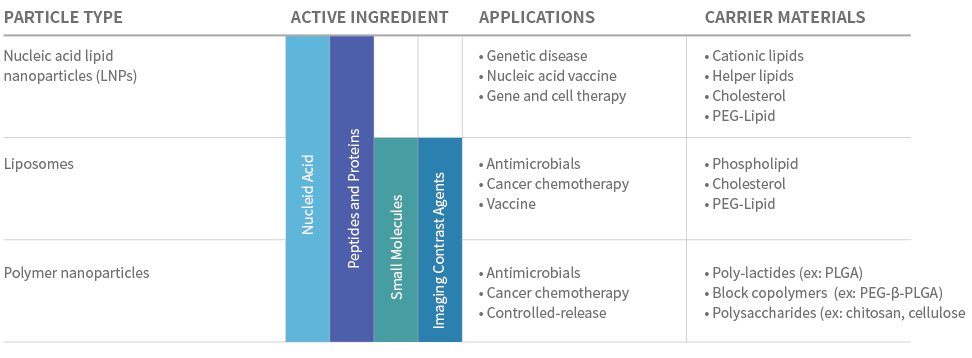
Microflow™ Applications
Previous:
Microflow S (Milliliter-scale)





